Before I get to that, though, I do want to mention the date is rapidly approaching for my CME course on Testosterone Therapy and Sexual Dysfunction. This 2-day course will take place February 16-17, 2024, in Orlando, Florida, now with the option to attend virtually. The course sold out last year, and it will sell out again even though we doubled the space for attendees. I encourage you to register soon if you are interested in attending.
We have an all-star faculty, including Abdul Traish, Mohit Khera, Michael Irwig, Kevin Flinn, Marianne Brandon, and Rachel Rubin. Here is the website for the course, which you can use to register: hmstestosteronecourse.com. I hope to see you there.
Now, on to the new TRAVERSE publication. My summary and comments on the initial TRAVERSE appears on this website as a video. Briefly, TRAVERSE was the largest placebo-controlled trial involving TTh, and was designed to be a cardiovascular trial, involving a large study population at high-risk for major adverse cardiovascular events (MACE). That study was written by Lincoff and co-workers and published in the New England Journal of Medicine (DOI: 10.1056/NEJMoa2215025).
The study population consisted of 5,246 men ages 45 to 80 years with either pre-existing cardiovascular (CV) disease or at high risk for CV disease. The bottom line was that MACE (consisting of non-fatal heart attack or stroke, or death due to a CV cause) occurred in 7.0% of the testosterone group and 7.3% of the placebo group. Clearly, there was no increased MACE risk with TTh.
This study had been mandated by the FDA to definitively answer the question as to whether TTh was associated with increased CV risk. Hopefully, this result should put to bed once and for all the concern about increased CV risk with TTh.
One additional valuable piece of information was that prostate cancer was diagnosed in 12 men in the testosterone group (0.5%) and 11 men in the placebo group (0.4%). These nearly identical rates should also put to bed the longstanding concern that TTh increased prostate cancer risk. Numerous prior studies – RCTs and observational studies – have failed to ever support increased prostate cancer risk, but this result from TRAVERSE should be the final nail in the coffin for this widely held yet incorrect concern. Testosterone therapy does not increase prostate cancer risk.
Now comes a second TRAVERSE paper by Pencina and colleagues, published in the Journal of Clinical Endocrinology and Metabolism (https://doi.org/10.1210/clinem/dgad484). In this subset of the original TRAVERSE study population men were required as before to have T levels less than 300 ng/dl (10.4 nmol) plus hypogonadal symptoms. There were 1161 men enrolled, 587 of whom were randomized to 1.62% testosterone gel and 574 randomized to placebo gel. Results showed TTh was associated with greater improvement in sexual activity, libido, and hypogonadal symptoms compared with placebo. Curiously, no significant difference between groups was noted for erectile function.
While improvement in hypogonadal symptoms, libido, and sexual activity will not be surprising to anyone with clinical experience with TTh or with familiarity with prior literature, these results still provide important confirmation of these benefits of TTh due to the nature of the study, its large size, and its rigorous design.
Prior to TRAVERSE the largest randomized clinical trial (RCT) that investigated the sexual benefits of TTh was the Testosterone Trials. The initial primary results of that study were also published in the New England Journal of Medicine in 2016 by Peter Snyder and colleagues. Results from that study also showed improvement in sexual activity, libido, and other hypogonadal symptoms, and contrary to results from TRAVERSE did show improvement in erection quality also.
The Testosterone Trials was a 12-month study of T gel vs placebo gel in 790 men who were 65 years or older. While the testosterone group in that study also demonstrated significantly greater improvements in sexual desire and activity compared to the placebo group, by the end of the study at 12 months there appeared to be a drop-off in benefits, raising questions about the durability of response to TTh. In addition, some critics of TTh commented that the smaller magnitude of effect noted at 12 months meant the benefits of TTh were questionable. In contrast, the benefits of TTh in TRAVERSE were robust and stable from 6 months through the last time point of 24 months.
The Testosterone Trials also showed a significantly greater improvement in erectile function among men that received testosterone compared with placebo. Why did this not show up in TRAVERSE? To me, the obvious explanation is the study population. The Testosterone Trials attempted to study a relatively healthy population and excluded men with known CV disease and at high-risk for MACE. This exclusion for the Testosterone Trials was actually the inclusion criteria for TRAVERSE! So TRAVERSE had a much higher rate of vascular disease in their population than the Testosterone Trials.
What does this have to do with erectile response to TTh? Men who have a vascular basis for their erectile dysfunction are highly unlikely to respond to TTh, whereas men whose ED is caused by testosterone deficiency will respond nicely to normalization of testosterone. There was a much greater likelihood that vascular causes accounted for ED in TRAVERSE compared with the Testosterone Trials, and so it is not surprising that improvement in erectile function was not noted in TRAVERSE.
TRAVERSE is a nice example of how the inclusion/exclusion criteria for a study population can have a large impact on results. There is some literature that argues TTh is not an effective treatment for ED. On a clinical basis this is obviously incorrect. For some men TTh restores all sexual symptoms, including ED, particularly among those who are younger and relatively healthy. In 1999, Abdul Traish and colleagues (https://doi.org/10.1210/endo.140.4.6655) showed in a rabbit model that castration abolished the erectile response to cavernosal nerve stimulation, and testosterone administration to castrated males restored it to baseline.
Here is a figure from that article, which clearly shows great impact of testosterone status on erectile function.
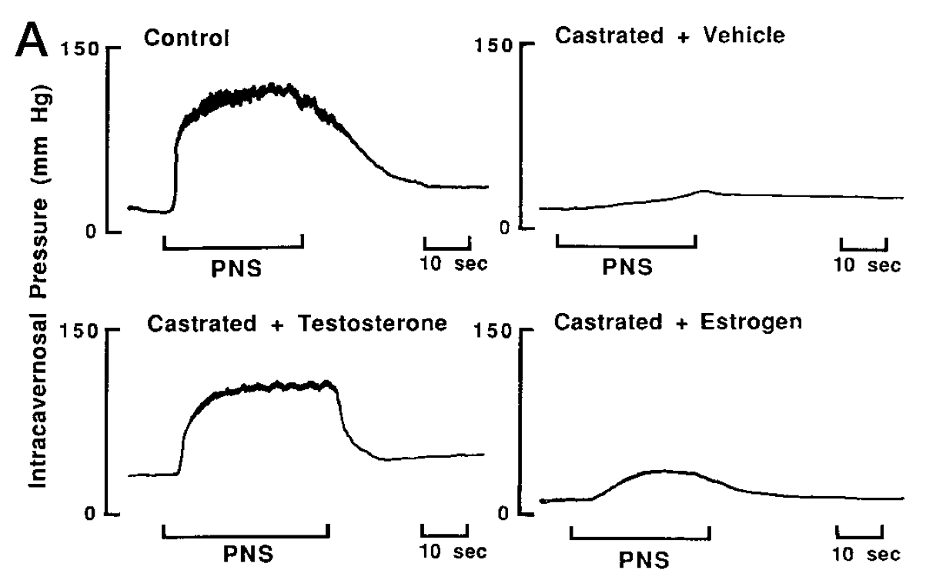
In my own patients, I would offer TTh alone (without a PDE5 inhibitor) to a man with T deficiency and symptoms that included weak erections. For many men this would be a complete success. In others the erectile response was insufficient, and I would then add in a PDE5 inhibitor.
One last comment about this latest TRAVERSE paper. I found it remarkable that the difference in sexual activity between the testosterone and placebo groups was 0.47 events per day at 12 months. This difference is 3 events per week. That’s huge! Note that sexual activity in this study included masturbation as well as intercourse with a partner.
Additional studies are expected to be published from the TRAVERSE data, and it will be interesting to see what new nuggets of information we will learn.
Best wishes to all for an enjoyable holiday season and new year, in whatever way you celebrate.

Abe Morgentaler, MD
